[TABLE="width: 480, align: center"]
[TR]
[TD="colspan: 2"][FONT=Helvetica, Arial]
[/TR]
[TR]
[TD="width: 90, align: center"][/TD]
[TD]
[/TD]
[/TR]
[TR]
[TD="colspan: 2"]
[/TR]
[TR]
[TD="width: 90, align: center"][/TD]
[TD]
[/TR]
[/TABLE]
[TR]
[TD="colspan: 2"][FONT=Helvetica, Arial]
THE NAMES OF MORE ORGANIC COMPOUNDS
[/FONT][FONT=Helvetica, Arial][/FONT]
[FONT=Helvetica, Arial]This page continues looking at the names of organic compounds containing chains of carbon atoms. It assumes that you have already looked at the introductory page covering compounds from alkanes to ketones.
[/FONT][/TD][/TR]
[TR]
[TD="width: 90, align: center"][/TD]
[TD]
[FONT=Helvetica, Arial][/FONT]
[FONT=Helvetica, Arial][HR][/HR][/FONT][FONT=Helvetica, Arial]Note: If you haven't already looked at that page, it would be a good idea to do so before you go on. The names on this second page aren't explained in quite as much detail as those on the introductory page - it assumes that you have already understood the main principles. If in doubt, follow this link first.
[/FONT]
[HR][/HR][/FONT]
[/TR]
[TR]
[TD="colspan: 2"]
[FONT=Helvetica, Arial][/FONT]
[FONT=Helvetica, Arial]More types of organic compound
[/FONT][FONT=Helvetica, Arial][/FONT]
[FONT=Helvetica, Arial]Carboxylic acidsCarboxylic acids contain the -COOH group, which is better written out in full as:
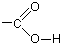 Carboxylic acids are shown by the ending oic acid. When you count the carbon chain, you have to remember to include the carbon in the -COOH group. That carbon is always thought of as number 1 in the chain.Example 1: Write the structural formula for 3-methylbutanoic acid.This is a four carbon acid with no carbon-carbon double bonds. There is a methyl group on the third carbon (counting the -COOH carbon as number 1).
Carboxylic acids are shown by the ending oic acid. When you count the carbon chain, you have to remember to include the carbon in the -COOH group. That carbon is always thought of as number 1 in the chain.Example 1: Write the structural formula for 3-methylbutanoic acid.This is a four carbon acid with no carbon-carbon double bonds. There is a methyl group on the third carbon (counting the -COOH carbon as number 1).
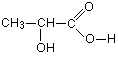
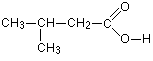 Example 2: Write the structural formula for 2-hydroxypropanoic acid.The hydroxy part of the name shows the presence of an -OH group. Normally, you would show that by the ending ol, but this time you can't because you've already got another ending. You are forced into this alternative way of describing it.
Example 2: Write the structural formula for 2-hydroxypropanoic acid.The hydroxy part of the name shows the presence of an -OH group. Normally, you would show that by the ending ol, but this time you can't because you've already got another ending. You are forced into this alternative way of describing it.
 The old name for 2-hydroxypropanoic acid is lactic acid. That name sounds more friendly, but is utterly useless when it comes to writing a formula for it. In the old days, you would have had to learn the formula rather than just working it out should you need it.Example 3: Write the structural formula for 2-chlorobut-3-enoic acid.This time, not only is there a chlorine attached to the chain, but the chain also contains a carbon-carbon double bond (en) starting on the number 3 carbon (counting the -COOH carbon as number 1).
The old name for 2-hydroxypropanoic acid is lactic acid. That name sounds more friendly, but is utterly useless when it comes to writing a formula for it. In the old days, you would have had to learn the formula rather than just working it out should you need it.Example 3: Write the structural formula for 2-chlorobut-3-enoic acid.This time, not only is there a chlorine attached to the chain, but the chain also contains a carbon-carbon double bond (en) starting on the number 3 carbon (counting the -COOH carbon as number 1).

Salts of carboxylic acidsExample: Write the structural formula for sodium propanoate.This is the sodium salt of propanoic acid - so start from that. Propanoic acid is a three carbon acid with no carbon-carbon double bonds.
 When the carboxylic acids form salts, the hydrogen in the -COOH group is replaced by a metal. Sodium propanoate is therefore:
When the carboxylic acids form salts, the hydrogen in the -COOH group is replaced by a metal. Sodium propanoate is therefore:
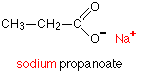 Notice that there is an ionic bond between the sodium and the propanoate group. Whatever you do, don't draw a line between the sodium and the oxygen. That would represent a covalent bond. It's wrong, and makes you look very incompetent in an exam!In a shortened version, sodium propanoate would be written CH[SUB]3[/SUB]CH[SUB]2[/SUB]COONa or, if you wanted to emphasise the ionic nature, as CH[SUB]3[/SUB]CH[SUB]2[/SUB]COO[SUP]-[/SUP] Na[SUP]+[/SUP].
Notice that there is an ionic bond between the sodium and the propanoate group. Whatever you do, don't draw a line between the sodium and the oxygen. That would represent a covalent bond. It's wrong, and makes you look very incompetent in an exam!In a shortened version, sodium propanoate would be written CH[SUB]3[/SUB]CH[SUB]2[/SUB]COONa or, if you wanted to emphasise the ionic nature, as CH[SUB]3[/SUB]CH[SUB]2[/SUB]COO[SUP]-[/SUP] Na[SUP]+[/SUP].
[/FONT][/TD]Salts of carboxylic acidsExample: Write the structural formula for sodium propanoate.This is the sodium salt of propanoic acid - so start from that. Propanoic acid is a three carbon acid with no carbon-carbon double bonds.
[/TR]
[TR]
[TD="width: 90, align: center"][/TD]
[TD]
[FONT=Helvetica, Arial][/FONT]
[FONT=Helvetica, Arial][HR][/HR]Note: The confusing thing about these salts (and even more so for the esters that are coming up next) is that they are named the wrong way round. In the formula, the sodium is at the end, but appears first in the name. Why?
[/FONT][FONT=Helvetica, Arial]Salts are always named with the metal first - think of sodium chloride or potassium iodide. So for consistency you would need to reverse the formula of sodium propanoate - NaOOCCH[SUB]2[/SUB]CH[SUB]3[/SUB]. But if you reverse the formula, you can't see immediately that it is related to propanoic acid. So you learn to live with the inconsistency.
[/FONT]
[HR][/HR][/FONT]
اگر مفید واقع شد بگید تا هم بقیش رو و هم سوالات رو براتون بذارم...
[/TD][/TR]
[/TABLE]
